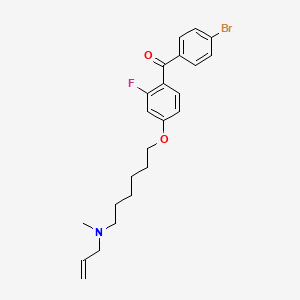
Ro 48-8071, 161582-11-2, [4-({6-[allyl(methyl)amino]hexyl}oxy)-2-fluorophenyl](4-bromophenyl)methanone, Ro-48-8071, Ro48-8071, YDR69X9Q9M, R048-8071, (4-bromophenyl)-[2-fluoro-4-[6-[methyl(prop-2-enyl)amino]hexoxy]phenyl]methanone, CHEMBL304858, (4-BROMOPHENYL)[3-FLUORO-4-[[6-(METHYL-2-PROPENYLAMINO)HEXYL]OXY]PHENYL]-METHANONE, 1gsz, (4-bromophenyl)[2-fluoro-4-({6-[methyl(prop-2-en-1-yl)amino]hexyl}oxy)phenyl]methanone, (4-[6-(allyl-methyl-amino)-hexyloxy]-2-fluoro-phenyl)-(4-bromo-phenyl)-methanone, [4-[6-(allyl-methyl-amino)-hexyloxy]-2-fluoro-phenyl]-(4-bromo-phenyl)-methanone, {4-[6-(Allyl-methyl-amino)-hexyloxy]-2-fluoro-phenyl}-(4-bromo-phenyl)-methanone, compound 2 [PMID: 22533316], Methanone, (4-bromophenyl)[2-fluoro-4-[[6-(methyl-2-propenylamino)hexyl]oxy]phenyl]-,(2E)-2-butenedioate, UNII-YDR69X9Q9M, (4'-(6-Allylmethylaminohexyloxy)-2'-fluorophenyl)-4-(4-bromophenyl)methanone fumarate, GTPL6710, SCHEMBL3674881, DTXSID90870077, CHEBI:101064, CMYCCJYVZIMDFU-UHFFFAOYSA-N, HMS3649J22, HMS3743C21, BCP30557, BDBM50128065, AKOS025294639, CS-3518, DB02016, NCGC00165878-01, NCGC00165878-02, HY-18630, NS00018037, SR-01000946731, J-009841, SR-01000946731-1, Q27088590, N-allyl-6-(4-(4-bromobenzoyl)-3-fluorophenoxy)-N-methylhexan-1-aminium, (4-bromophenyl)-[2-fluoro-4-[6-(methyl-prop-2-enylamino)hexoxy]phenyl]methanone, (4-Bromophenyl)[2-fluoro-4-[[6-(methyl-2-propenylamino)hexyl]oxy]phenyl]methanone, [4-[6-[allyl(methyl)amino]hexoxy]-2-fluoro-phenyl]-(4-bromophenyl)methanone, Methanone, (4-bromophenyl)(2-fluoro-4-((6-(methyl-2-propenylamino)hexyl)oxy)phenyl)-, Methanone, (4-bromophenyl)[2-fluoro-4-[[6-(methyl-2-propen-1-ylamino)hexyl]oxy]phenyl]-, Ro 488071; Ro488071; Ro-488071; Ro 488071; Ro-488071